How to discuss what’s next after 2 or more diffuse large B-cell lymphoma (DLBCL) therapies
Diffuse Large B-Cell Lymphoma (DLBCL) — A type of B-cell non-Hodgkin lymphoma (cancer of the immune system) that is usually aggressive (fast-growing). It is the most common type of non-Hodgkin lymphoma, and is marked by rapidly growing tumors in the lymph nodes, spleen, liver, bone marrow, or other organs.
Your doctor may explain your options with complicated medical terms. They may use words you’re hearing for the first time or talk about ideas that may be difficult to understand in the moment.
If you have already tried 2 or more past therapies for relapsed/refractory (R/R) DLBCL, but need your next one, a lot of questions may be coming up. If therapies have not worked or are no longer working, it may be difficult to believe a new therapy will. If the cancer continued to grow after past therapies worked, you may also feel let down.
At this time, discussions with your doctor about your options are crucial.
If other therapies have worked, but the cancer returned,
or no other therapies have worked—there’s still hope that another option might help
If you’re considering, or are on ZYNLONTA® therapy, the questions below can help guide future conversations with your doctor.
Take notes and ask as many questions as you need. This way, you can understand more about your therapy.
Ask about whether ZYNLONTA® is right for you
Why you should ask: ZYNLONTA® may not be right for everyone. Ask your doctor if ZYNLONTA® could be the next step for your specific type of R/R DLBCL.
Your doctor will consider a number of factors about your personal health.
ZYNLONTA® may be an option after most DLBCL therapies.
Some adults treated with ZYNLONTA® were able to go on to other therapies
Ask about relapsed vs refractory DLBCL
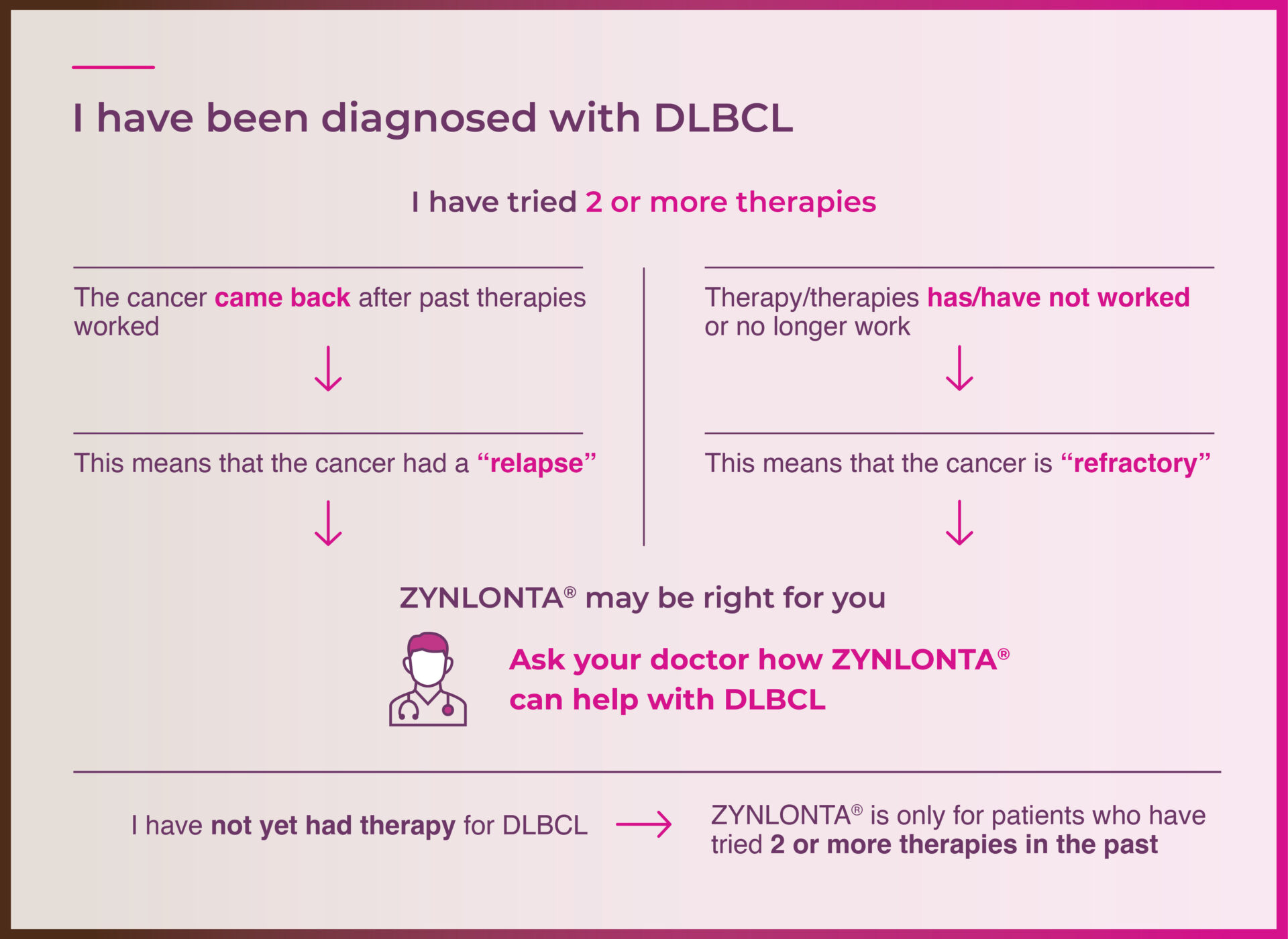
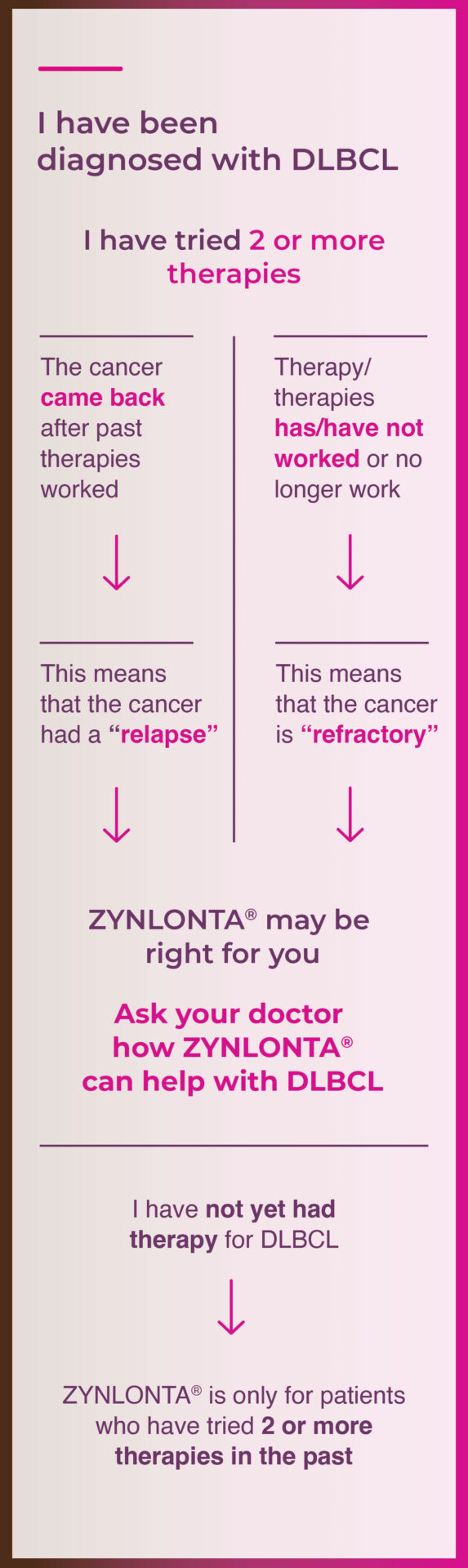
Something your doctor will take into account is whether the cancer has relapsed or is refractory. Use the flow chart below to figure out where your cancer falls.
Why you should ask: There are 2 ways to think about how DLBCL has reacted to past therapy. Use the flow chart below to understand more about what your doctor will want to know.
Ask about therapy information
Why you should ask: Sometimes talking points may not readily come to mind. But these questions are made to open up the conversation with your doctor about ZYNLONTA®. Feel free to download them.
- What kind of infusion is ZYNLONTA®?
- Is ZYNLONTA® right for my specific condition?
- How well does ZYNLONTA® work?
- How is ZYNLONTA® given?
- How long does each treatment take? How often is ZYNLONTA® given?
- When can I start seeing results with ZYNLONTA®? How will I know it’s working?
- Before taking ZYNLONTA®: Will I need to take any medications? Do I need to do anything else?
- How often will blood tests be done?
- Does it matter what therapy I was on before?
- Is the therapy different if I have relapsed?
- Would my therapy be different if the cancer had never responded to therapy before?
- What are possible side effects I should look out for?
- What are more serious side effects and which are more common?
- When should I contact your office about side effects?
- Is there anything I should avoid taking or doing while on ZYNLONTA®?
- What are some trusted sources that can help me understand my condition?
- What are the best ways to reach you when I have questions?
- How long will I be on ZYNLONTA®?